Megan Munsie, The University of Melbourne and Melissa Little, Murdoch Children’s Research Institute
The International Society for Stem Cell Research (ISSCR) today released updated guidelines for stem cell research and its translation to medicine.
Developed in response to recent scientific and clinical advances, the revised guidelines provide a series of detailed and practical recommendations that set out global standards for how these emerging technologies should be harnessed.
Stem cell research has huge potential — it could help pave the way for new therapies for ailments ranging from Parkinson’s disease to childhood kidney failure. But scientific advances in this field can present unique ethical and policy issues beyond that seen in other areas of medical research.
The science is advancing at breakneck pace. Just in the past couple of months, we have seen model human embryos grown from skin cells, and the creation of human-monkey embryos for use in research.
The ISSCR has long recognised the need to set clear ethical boundaries for stem cell research. Previous guidelines have provided advice on techniques such as the use of human embryos to create stem cells, and set the required standards when using these technologies to create new medicines.
They have also explicitly banned certain practices, such as reproductive cloning and the sale of unproven therapies that claim to be made of stem cells.
The 2021 guidelines — an update on the previous version, released in 2016 — aim to set standards for the many recent advances in stem cell and human embryo research. These include “chimeric” embryos containing cells from humans and other animals, “organoids” grown from stem cells to create tissue that resembles particular human organs, and “models” of human embryos — arrangements of human cells that mimic the early stages of embryo development.
So what’s new?
The guidelines contain a clear requirement for certain new stem cell research approaches only to be conducted after a specialised review process. This review should be independent of the researchers, and include community members as well as people with expertise in the relevant science, ethics and law.
This is beyond what is typically required by a university or research institute where medical research is conducted. Besides evaluating the merit of the proposed research, the new reviews should also consider whether there are alternative ways to do the research, the source of stem cells and how they were obtained, and the minimum time required to reach the research goals, particularly in relation to human embryo and related research.
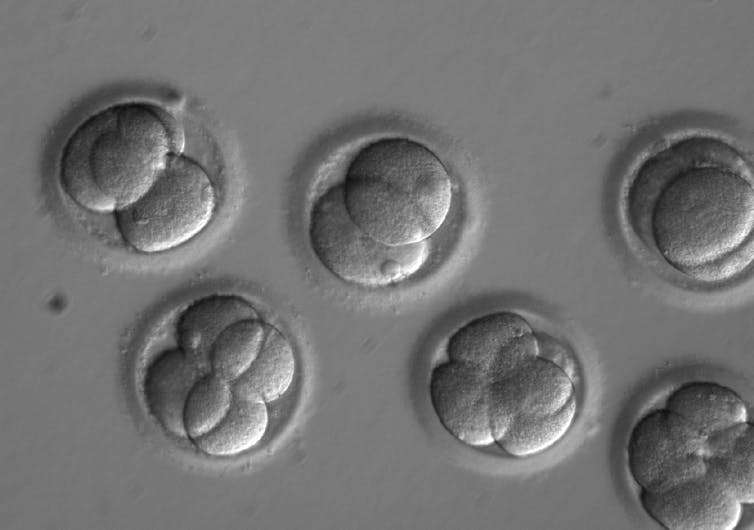
Specialised review is not a new concept. The previous guidelines required it when researchers made stem cells from human embryos or sought to culture human embryos in the lab. But now researchers will now also be required to seek higher review when they create model embryos such as blastoids, or study the development of animal-human embryos in animal wombs.
Researchers developing new therapies for mitochondrial disease will also be required to seek higher-level review before attempting to transfer to the uterus of a woman human embryos in which affected mitochondria (a part of the cell’s energy-production apparatus) have been replaced.
Importantly, the revised guidelines also clearly rule out certain activities. These continue to include reproductive cloning and attempts to form a pregnancy in a woman from genetically “edited” human embryos or from model embryos made from stem cells. Prohibited activities also now include using eggs and sperm made from human stem cells for reproduction, or transferring a human-animal chimeric embryo into the uterus of a woman or an ape.
The guidelines also call for a public conversation about whether we should allow limited lab research on human embryos beyond the existing limit of 14 days’ development. Historically, it has not been possible to support human embryonic development outside the body beyond this stage. However, recent advances in human embryo culture raise the possibility that this may now be technically feasible.
Extending the amount of time in culture – in terms of days – could potentially yield new treatments for developmental conditions or infertility, but will also raise concerns about whether possible benefits justify this research. Any decisions to overturn this long-held signpost would need to be carefully deliberated and take into consideration existing law, community values and discussion around what the new limit should be.
The revised guidelines also reinforce the need for informed consent for the collection of human material and participation in stem cell clinical trials, and reiterate that no new stem cell treatment should be made available before it is tested for safety and effectiveness in well-designed and publicly visible clinical trials. The ISSCR continues to condemn the commercial use of unproven stem cell treatments.
Why do these guidelines matter?
While stem cell science holds much promise, it is paramount that research is scientifically and ethically rigorous, with appropriate oversight, transparency and public accountability.
The fact these guidelines are driven by experts – including stem cell scientists, doctors, ethicists, lawyers and industry representatives – from across 14 countries indicates a deep sense of responsibility and integrity within the research community, and a desire to ensure science remains in step with community values.
However, these guidelines are recommendations, not laws.
Researchers will need to abide by their respective national or state regulations and ethical standards. Some countries already have regulatory frameworks that are consistent with the new recommendations. In other places there is no national guidance around laboratory and clinical stem cell research at all, or existing law touches on some but not all of the emerging applications of stem cell research.
For example, in Australia there is already an established pathway for higher-level review of embryo models created from stem cells. However, the same legislation currently bans any attempt to use mitochondrial transfer techniques to create embryos for research or to achieve a pregnancy – both of which are permissible under the new ISSCR guidelines.
Rather than attempting to impose a set of hard-and-fast rules on an ever-evolving research field, the new guidelines attempt to address emerging issues and drive important discussions at domestic level. Ultimately, it is the public and the regulators who will need to set the standards.
Megan Munsie, Head Ethics, Education & Policy in Stem Cell Science and Convener of Stem Cells Australia, The University of Melbourne and Melissa Little, Theme Director, Cell Biology, Murdoch Children’s Research Institute
This article is republished from The Conversation under a Creative Commons license. Read the original article.












